Provided by:

Implant and restoration success has become a predictable treatment modality and has become a common aspect of dental practices1-3. As the number of patients selecting dental implants as a treatment option continues to grow, the dental team must accept the challenges of maintaining these sometimes complex restorations. This is important in all practices whether DSO or individual doctors offices. Maintenance of these falls both on the patient who needs to practice daily homecare, but also practitioners (dentists and hygienists) who will see these on regular recall prophy appointments.
Proper monitoring and maintenance is essential to ensure the longevity of the dental implant and its associated restoration through a combination of appropriate professional care and effective patient oral hygiene 4,5. The value of using conventional periodontal parameters to determine peri-implant health is not clearly evident in the literature4. Therefore, it is paramount that the dental implant team understands the similarities and distinctions between the dental implant and the natural tooth. Subsequently, by examining the similarities and differences between a natural tooth and a dental implant, basic guidelines can be provided for maintaining the long-term health of the dental implant.
Direct anchorage of alveolar bone to a dental implant body provides a foundation to support a prosthesis and transmits occlusal forces to the alveolar bone. This is the definition of osseointegration6. With the increased acceptance of dental implants as a viable treatment option for the restoration of a partially edentulous or edentulous mouth, the dental team is faced with maintaining and educating those patients.
Recently, the focus of implant dentistry has changed from obtaining osseointegration, which is highly predictable, to the log-term maintenance health of the peri-implant hard and soft tissues. This can be achieved through appropriate professional care, patient cooperation, and effective home care7. Patients must accept the responsibility for being co-therapists in maintenance therapy, so the dental team essentially must screen the potential implant patient. Diagnosis and treatment planning based on a risk-benefit analysis should be performed subsequent to a thorough medical, dental, head-and-neck, psychological, tempormandibular disorder and radiographic examination.8
There is convincing evidence that bacterial plaque not only leads to gingivitis and periodontitis9, but also can induce the development of peri-implantitis 10. Thus, personal oral hygiene must begin at the time of dental implant placement and should be modified using various adjective aids for oral hygiene to effectively clean the altered morphology of the peri-implant region before, during, and after implant placement. For instance, interproximal brushes can penetrate up to 3mm into a gingival sulcus or pocket and may effectively clean the peri-implant sulcus 11. In addition to mechanical plaque control, daily rinses using 0.1% chlorhexidine gluconate or Listerine 12 provides a welcome adjunct.
Hygiene with dental implants is so tedious and critical to their long-term success that the patient and dental professional must exercise considerable effort. During the maintenance visit, the dental professional should concentrate on the peri-implant tissue margin, implant body, prosthetic abutment to implant collar connection, and the prosthesis13.
Clinical inspection for signs of inflammation, ie. bleeding on probing, exudate, mobility, probe-able pockets, and a radiographic evaluation of the peri-implant bony housing still remains the standard mode for evaluating the long-term status of endosseous dental implants. For instance, successful and stable endosseous dental implants exhibit no mobility. But, if there is clinically perceptible mobility, then subsequent to radiographic evaluation of the implant and its surrounding bony housing, the abutment retaining screw14, and/or prosthetic abutment collar interface should be examined for looseness or breakage.
All these modes of clinical assessment are used routinely, except for periodontal probing around peri-implant tissues that appear to be in a state of good health. The baseline data and data from subsequent recare visits should be recorded in the daily progress notes to properly assess the peri-implant status longitudinally.
Subsequent to a thorough intraoral examination, unless there is visual evidence of soft tissue changes, ie. inflammation of peri-implant tissue with even slight attachment loss or mucositis, routine probing of the peri-implant tissue should not be performed.
Usually during the first year subsequent to restoring dental implants, a 3-month recare schedule should be implemented, especially if the patient lost teeth because of periodontal disease. But if after 12 months, the patient’s implants are stable and peri-implant tissues are healthy, then a 4-6 month recare regimen can be implemented 15. However, be cognizant of each patient’s level of home care effectiveness, systemic health, and periodontal status of the peri-implant tissue when determining these recare intervals.
With dental implant patients, the dental professional must evaluate the prosthetic components for plaque, calculus, and the stability of the implant abutment. Radiographs of dental implants should be taken every 12 to 18 months during these maintenance visits16. For dental implant restorations that are screw retained, the dental professional needs to remove the prosthesis at least once a year to more easily assess the status of the peri-implant’s hard and soft tissues, the existence of acceptable mobility of the prosthetic components or the implant fixture itself, and the patient’s level of home care effectiveness17. Remember that the presence of any symptoms of infection, radiographic evidence of peri-implant bone loss, and/or neuropathies may be indicative of an ailing or failing implant 18.
Implants vs natural teeth
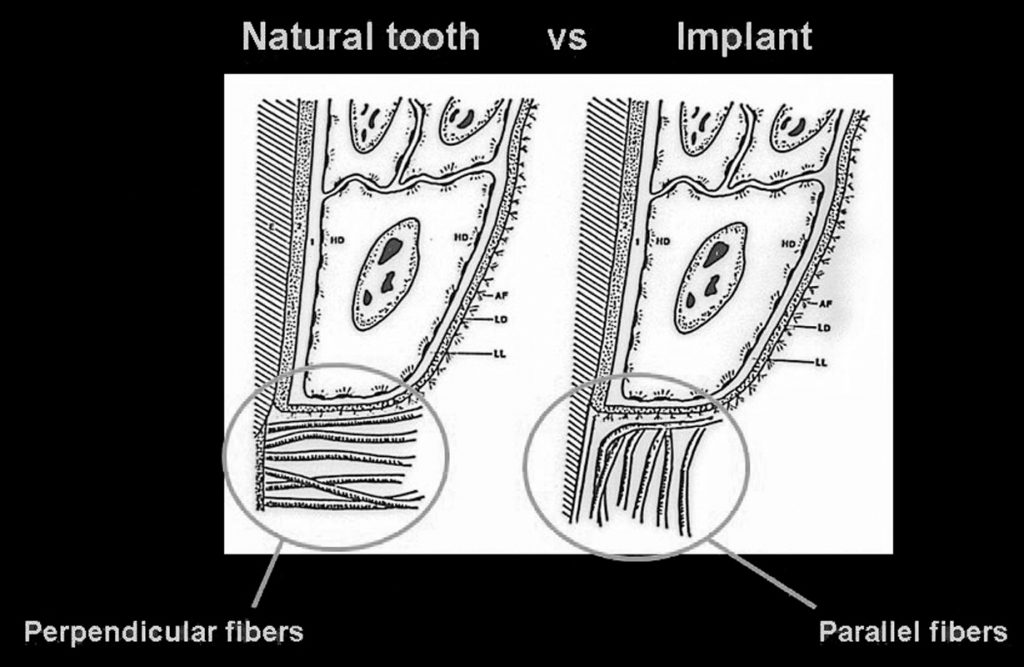
It is essential to understand the periodontal relationship between the gingiva and the structure it attaches to be it a natural tooth or an implant. (Figure 1 and 2) The fiber orientation of the gingival cuff around a natural tooth attaches perpendicular to the long axis of the tooth. (figure 3) This acts as a barrier when insertion of a periodontal probe within the sulcus. The probe tip advances apically till the tip contacts the perpendicular fibers and is halted. This orientation is not seen around implants. With an implant the gingival fiber orientation is parallel to the implants long axis. (figure 4) When a periodontal probe is inserted into the sulcus around an implant the probe tip advances passing between the fibers of the gingival cuff till the crestal bone prevents it from further advancement.
The peri-implant mucosal seal may be less effective barrier to bacterial plaque than the periodontium around a natural tooth, tissue attachment 19. There is less vasculature in the gingival tissue surrounding dental implants compared to natural teeth. This reduced vascularity concomitant with parallel-oriented collagen fibers adjacent to the body of any dental implant make dental implants more vulnerable to bacterial insult 20. During recare appointments, peri-implant periodontal probing should be performed only where signs of infection are present, ie. exudate, swelling, bleeding on probing, inflamed peri-implant soft tissue, and/or radiographic evidence of peri-implant alveolar bone loss. Lastly, routine periodontal probing of dental implants should not be performed, because this procedure could damage the weak epithelial attachment around dental implants, possibly creating a pathway for the ingress of periodontal pathogens21. Commercially available plastic probes should be used when investigating the crevicular depth around dental implants. The probing depth around dental implants may be related closely to the thickness and type of mucosa surrounding the implant. A healthy peri-implant sulcus has been reported to range from 1.3 to 3.8mm, which is greater than those depths reported for natural teeth 22. In essence, the best indicator for evaluating an unhealthy site would be probing data gathered longitudinally 23.
For all of these reasons, personal home care and consistent professional maintenance have proven to be critical to the success and longevity of endosseous dental implants. This is especially true in an environment with adjacent natural teeth, which if affected by periodontal disease, could act as a reservoir for pathogenic bacteria, ie. gram-negative anaerobic rods, and seed the peri-implant sulcus 24.
The physical characteristics of the peri-implant soft tissue are the focus of all oral hygiene instruction. The presence or absence of keratinized tissue in this critical area has not been unequivocally documented to state that peri-implant tissues are more vulnerable to the ingress of pathogenic bacteria with or without keratinized tissue being present around dental implants. However, the ability of the patient to maintain good home care around dental implants is facilitated by the presence of keratinized tissue surrounding implants. Thus, if a patient has no keratinized tissue around an implant, and a pull from a frenum or a chronic peri-implant mucositis exists, then placement of a soft tissue autogenous or alloplastic connective tissue graft is recommended to facilitate proper mechanical oral hygiene maintenance.25
Specific criteria for obtaining clinical data around dental implants that would allow proper monitoring and detect early possible failure of osseointegrated dental implants has not been clearly defined. Presently, the presence of mobility is the best indicator for diagnosis of implant failure. As opposed to natural teeth, dental implants exhibit minimal clinically undetectable movement because of the absence of a periodontal ligament. Therefore, healthy implants should appear non-mobile, even in the presence of peri-implant bone loss, if an adequate amount of supporting alveolar bone still exists 26.
When monitoring the health of the peri-implant soft tissues, the practitioner should be cognizant of changes in soft tissue color, contour, and consistency. The presence of a fistulous tract could indicate the presence of a pathologic process or implant fracture.
BLEEDING
There is controversy in the literature as to the accuracy and significance of bleeding upon probing around dental implants. Presently, the literature advocates the use of bleeding on probing as an indicator of peri-implant disease, because it can occur prior to histologic signs of inflammation or concurrently with other signs of implant failure, ie. bone loss. However, as previously mentioned, routine probing is not recommended.
RADIOGRAPHIC EVALUATION
Radiographic interpretation is one of the most useful clinical parameters for evaluating the status of an endosseous dental implant. Invasion of biologic width, predictable remodeling, or so-called saucerization, is an average marginal bone loss of 1.5mm during the first year following prosthetic rehabilitation followed by an average of 0.2mm of vertical bone loss every subsequent year. Thus, progressive bone loss around a dental implant that exceeds these averages may be indicative of an ailing or failing implant. Lastly, during radiographic evaluation, no evidence of a peri-implant radiolucency should be found, because such a rarefaction usually indicates infection or failure to osseointegration 27.
PROFESSIONAL CLEANING INSTRUMENTATION
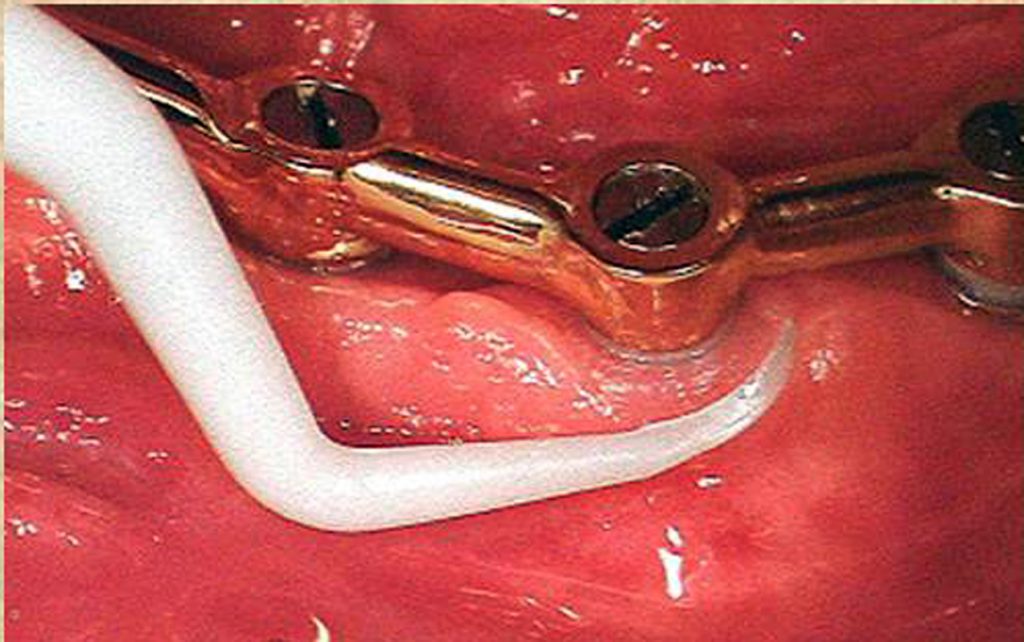
Instruments made of metal, such as stainless steel, should be limited to natural teeth and not to be used to probe or scale dental implants. The rationale for this well-documented and spoken conclusion is that this metal is so hard it can scratch, contaminate, or cause a galvanic reaction at the implant-abutment interface 28.
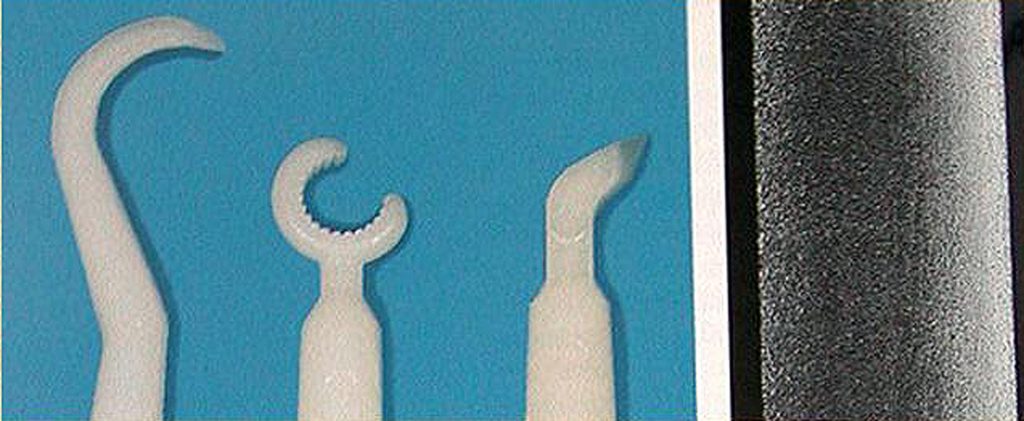
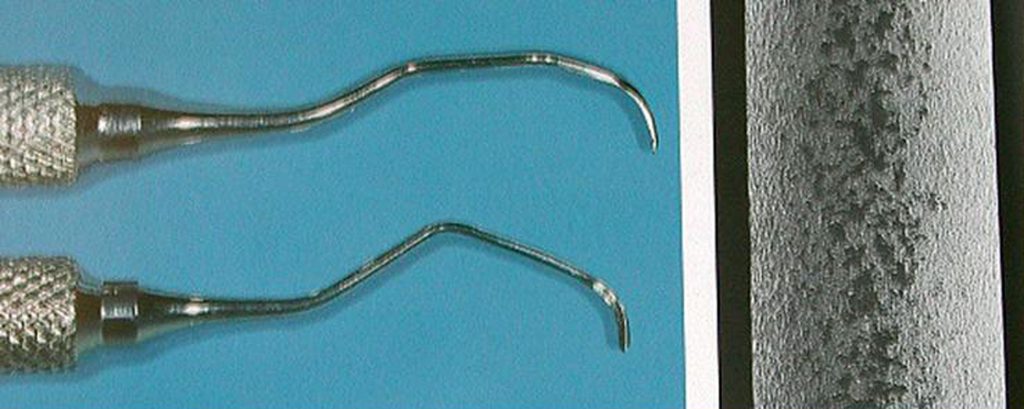
Ideally, hand periodontal scalers for cleaning dental implants can be plastic, Teflon, gold-plated, or made of wood (Figs 5 and 6) 29. When using gold-plated curettes, the manufacturer recommends not sharpening these hygiene instruments, as the gold surface could be chipped exposing the hand metal underneath this coating. Stainless steel scaling instruments may abraid the implant surface, stripping off any surface treatment such as hydroxyapatite (HA) as the instruments hardness is greater then the titanium alloy the implant is fabricated from. (Fig. 7)
Other cleaning armamentarium contraindicated for use with dental implants are air powder abrasive units, flour or pumice for polishing, and sonic and ultrasonic scaling units 30. Ultrasonic, piezio or sonic scaler tips may mar the implants surface leading to micro-roughness and plaque accumulation. The stainless steel tip may also lead to gouging of the implants polished collar. (Fig. 8) However, some clinicians advocate using a sonic instrument with a plastic sleeve over the tip for scaling dental implants. Air powder polishing units may also damage the implant surface and should be avoided during hygiene appointments. (Fig. 9) Even the use of baking soda powder in these units may strip off any surface coating on the implant. Additionally, the air pressure may detach the soft tissue connection with the coronal of the implant leading to emphysema.
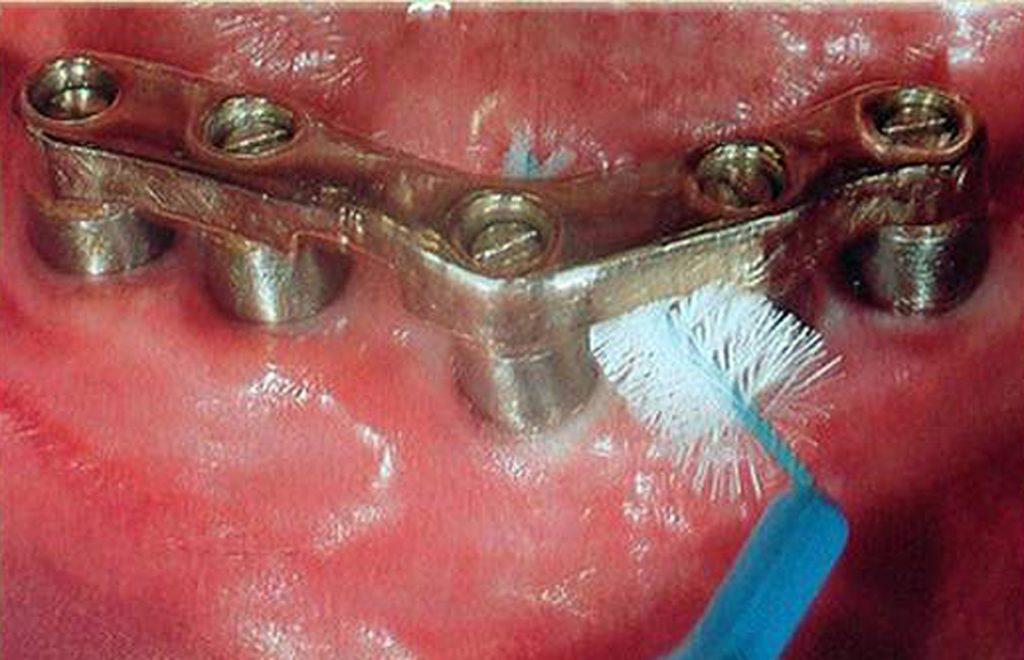
Titanium or titanium alloy surfaces of dental implants can be polished using a rubber cup along with a nonabrasive polishing paste or a gauze strip with tin oxide. Not only is the hygiene armamentarium important, but also are the home care techniques used to maintain endosseous dental implants. Patients should be taught the modified bass technique of brushing using a medium-sized head, soft-bristled toothbrush. The use of interdental brushes should be used by implant patients after being shown their proper use. The plastic-coated wire brush is the only type to be used with dental implants to clean and not scratch the implant surface (Fig. 10).
Recently, automated mechanical toothbrushes have been advocated as a daily mode of tooth cleansing. These devices may be a rotary, circular, or sonic type. With these home care instruments, the key to their effectiveness is proper instruction on their use and then diligent daily use by the implant patient.
As with natural dentition, adjective cleaning aids such as flossing are still valuable. As with dentate patients, an implant patient’s home care requirements should be individually tailored according to each patient’s needs. Individual needs are based on the location and angulation of the dental implants, the position and length of transmucosal abutments, the type of prosthesis, and the dexterity of each patient.
The other popularized type of cleansing device is the use of oral irrigators with or without the addition of antimicrobial solutions. Also, oral rinses with antimicrobial properties such as Listerine or chlorhexidine have been widely advocated throughout the literature 31-33.
Conclusion
During the infancy years of dental implantology, the emphasis for long-term success of osseointegrated implants was the surgical phase of dental implantology. In the years that followed, the emphasis for success had switched from a purely surgical influence to focusing more on the proper fixture placement that would be dictated by the prosthetic and aesthetic needs of each particular case.
In more recent years, the dental professional has recognized professional implant maintenance and diligent patient home care as two critical factors for the long-term success of dental implants. The microbiota and clinical presentation of peri-implantitis is the same as periodontitis around a natural tooth. With proper care in-office when rendering recall prophy treatment as outlined we can avoid surface damage to the implant that may lead to peri-implantitis related to increased plaque accumulation potential to the rougher surface. Additionally, as the fiber orientation around natural teeth and implants is different avoidance of routine perio probing is advised to avoid with implants, as this could be the initiation factor to breakdown of the periodontal connection.
DSO practices will see patients who have either been treated for implants in the practice or those patients who have implants placed in the past that will require ongoing oral hygiene care. Understanding the differences between care of natural teeth and implants will aid your patients in maintaining their implants long-term and minimizing potential issues that may result.
Author information:
Dr. Kurtzman is in private general practice in Silver Spring, Maryland, USA and a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry. He can be reached at [email protected].


References
1. Adell R, Lekholm U, Rockler B, et al. A 15 year study of osseoinegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387-416.
2. Cox JF, Zarb GA. The logitudinal clinical efficacy of osseointegrated dental implants: a 3 year report.Int J oral Maxillofac Implants. 1987;2:91-100.
3. Albrektsson T, Branemark P,Hansson HA, et al. Osseointegrated titanium implants. Acta Orthop Scand. 1981;52:155.
4. Orton GS, Steele DL, Wolinsky LE. The dental professional’s role in monitoring and maintenance of tissue-integrated prostheses. Int J Oral axillofac Implants. 1989;4(4):305-310.
5. Bauman GR, Mills M, Rapley JW, et al. Clinical parameters of evaluation during implant maintenance. Int J Oral Maxillofac Implants. 1992;7(2);220-227.
6. Rateischak KH, Wolf HF, eds Color Atlas of Dental Medicine; Implantology. Stuttgart, NY: Thieme Medical Publishers; 1995:305-316.
7. Meffert RM, Langer4 B, Fritz ME. Dental Implants: a review. J Periodontol. 1992;63(11):859-870.
8. Meffert RM. Contemporary Implant Dentistry. Carl E. Misch, ed. st Louis, Mo: Mosby Year Book; 1993:chap33.
9. Warrer K, Buser D, Lang NP, et al. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa: an experimental study in monkeys. Clin Oral implant Res. 1995;6:131-138.
10. Lang NP, Karring T. Proceedings of the 1st European Workshop on Periodontology. Chicago, IL: Quintessence; 1994.
11. Balshi TJ. Hygiene maintenance procedures for patients treated with the tissue-integrated prothesis
(osseointegration). Quintessence 1986;17(2):95-102.
12. Ciancio SG, Lauciello C, Shibley O. et al. The effect of an antiseptic mouthrinse on implant maintenance: plaque and peri-implant gingival tissues. J. Periodontol. 1995;66(11):962-965.
13. Garg AK. Practical Implant Dentistry. Dallas, TX: Taylor Publishing Co: 1995:111-115.
14. Lekholm U, Ericsson I, Adell R, et al.: The condition of the soft tissues at tooth and fixture abutments supporting fixed bridges: a microbiological and histological study. J Clin Periodontol 1986;13:558-562.
15. American Academy of Periodontology. Annals of Periodontology. 1996 World Workshop in Periodontics. 1996:1(1):816-820.
16. Baumgarten HS, Chiche GJ.: Diagnosis and evaluation of complications and failures associated with osseointegrated implants. Compend Contin Educ Dent. 1995 Aug;16(8):814-822.
17. Meffert RM. In the spotlight: Implantology and the dental hygienist’s role. J Prac Hygiene. 1995; September:12-14.
18. Meffert RM.: How to treat ailing and failing implants. Implant Dent. 1992 Spring;1(1):25-33.
19. Weyant RJ.: Characteristics associated with the loss and peri-implant tissue health of endosseous dental implants. Int J Oral Maxillofac Implants. 1994 Jan-Feb;9(1):95-102.
20. Nevins M, Langer B.: The successful use of osseointegrated implants for the treatment of the recalcitrant periodontal patient. J Periodontol. 1995 Feb;66(2):150-7.
21. Lang NP, Wetzel AC, Stich H, Caffesse RG.: Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res. 1994 Dec;5(4):191-201.
22.
van
Steenberghe D, Klinge B, Linden U, Quirynen M, Herrmann I, Garpland C.: Periodontal indices around natural and titanium abutments:
a longitudinal multicenter study.
J Periodontol. 1993 Jun;64(6):538-41.
23. Quirynen M, van Steenberghe D, Jacobs R, Schotte A, Darius P.: The reliability of pocket probing around screw-type implants. Clin Oral Implants Res. 1991 Oct-Dec;2(4):186-92.
24. Mombelli A, Marxer M, Gaberthuel T, Grunder U, Lang NP.: The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995 Feb;22(2):124-30.
25. Artzi Z, Tal H, Moses O, Kozlovsky A.: Mucosal considerations for osseointegrated implants. J Prosthet Dent. 1993 Nov;70(5):427-32.
26. Papaioannou W, Quirynen M, Nys M, van Steenberghe D.: The effect of periodontal parameters on the subgingival microbiota around implants. Clin Oral Implants Res. 1995 Dec;6(4):197-204.
27. Apse P, Ellen RP, Overall CM, Zarb GA.: Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: a comparison of sites in edentulous and partially edentulous patients. J Periodontal Res. 1989 Mar;24(2):96-105.
28. Speelman JA, Collaert B, Klinge B.: Evaluation of different methods to clean titanium abutments. A scanning electron microscopic study. Clin Oral Implants Res. 1992 Sep;3(3):120-7.
29. Gantes BG, Nilveus R.: The effects of different hygiene instruments on titanium surfaces: SEM observations. Int J Periodontics Restorative Dent. 1991;11(3):225-39.
30. Rapley JW, Swan RH, Hallmon WW, et al. The oral hygiene instruments and materials on titanium implant abutments. Int J oral Maxillofac Implants. 1990;5:47-52.
31. Mombelli A, Lang NP.: Antimicrobial treatment of peri-implant infections. Clin Oral Implants Res. 1992 Dec;3(4):162-8.
32. Ciancio S.: Expanded and future uses of mouthrinses. J Am Dent Assoc. 1994 Aug;125 Suppl 2:29S-32S.
33. Garg AK, Duarte F, Funari K.: Hygienic maintenance of dental implants: the key to success. J Pract Hygiene. 1997;6(2):13-20.











